
Are we at risk of a disaster movie becoming reality?
Almost all of us have required antibiotics at some point in our lives, but do we really understand what they do? How they work? More importantly, do you know that we could be much closer to losing their amazing protective power altogether? To explain why, let me ask you a question…
Do you remember a time before flat screen TVs? How amazing they were when they first arrived? How about mobile phones, or the internet?
Over time, we adjust and become used to new things. Imagine going back to a big, chunky TV now, or having to wait until you got home or to a phone box to ring someone. Time has a habit of making us forget how difficult things used to be, generating a complacency that can put that more comfortable way of life at risk. The same is true with medicine.
Antibiotics, in one form or another, have existed since the early 20th century. The most prominent early example is a man called Alexander Fleming’s apparent “accidental” discovery of a well-known antibiotic. The story goes that Fleming left out a small dish of Staphylococcus bacteria (often a cause of food poisoning) on his lab bench before leaving for the summer, where it was by chance contaminated by mould. When Fleming returned, he investigated further, and found that the mould was stopping the growth of the bacteria by breaking them down. The mould turned out to be Penicillium notatum, the byproduct of which Fleming branded the now famous ‘Penicillin’.
So let your flatmates//spouse/pets know that if you leave plates of unfinished food on the kitchen top for weeks to gather mould, you are actually attempting to discover a new and potentially lifesaving treatment. Do not be perturbed by the modern pharmaceuticals industry either. Sure, they have hundreds of millions of pounds to potentially pour into researching new antibiotic treatments, but there is not as much progress there as you’d expect. In fact, there have been almost no new antibiotics produced in the last 30 years. Why?

“I’ll throw it away first thing tomorrow, I swear!” – The words of a genius biologist.
The worrying answer is that antibiotic use is seen as a big risk for healthcare professionals and policy makers. There is not much money to be made in something that hardly ever gets used. But why is antibiotic use so sparse?
Throughout modern medicine, antibiotics efficiency in dealing with bacterial infection has never been in question, but with extended use on such a large scale, a biological battle began to rage, unseen by most. A battle of resistance.
A rare picture of the bacteria known as ‘flagstaph-ylococcus’
Antibiotics, from penicillin to the most recently discovered, were becoming non-functional, and the battle still rages on today.
How did this happen? Surely what killed bacteria 50 years ago should have exactly the same effect then as it does now?
For an explanation, we need to go to the frontlines of the war: the inside of your body. Like actual war, the technology has progressed, and the humble penicillin takes on new shapes, with a whole family of antibiotics to its name (with well-known examples including amoxicillin and ampicillin.)

“Fight not to scale, as you’ll see soon”
Bacteria are the main cause of infection in broken skin, mainly because they’re already all over your skin before it breaks (don’t think about it too much). They have 2 layers of protection: an outer thick cell wall, made of proteins known as peptidoglycans, as well as a thin and wobbly inner cell membrane. The wall gives them a solid shape whilst also protecting from damage. It is a key part of their structure, without which the bacteria will die.

There are other smaller bits of the cell wall, but peptidoglycans are the thing that gives it strength and structure.
The reason the penicillin family of antibiotics has been so successful in treating these sorts of infections is that it can block the production of this cell wall. So how does it do this?
Here is where the art of microbial war becomes interesting. Penicillin does not attack this very sturdy cell wall head-on, instead, it sneaks into the bacteria itself, behind enemy lines, where it finds an enzyme known as transpeptidase. These enzymes are the builders and repairers of the cell wall. Think of them as the ones who put the mortar between the bricks that are peptidoglycans.

“It’s transpeptidase time” just doesn’t have the same ring to it.
Penicillin binds to these transpeptidases, blocking them from being able to maintain the walls strength as the bacteria grows. This results in a breakdown of the wall, and a rather embarrassed and weak bacteria.
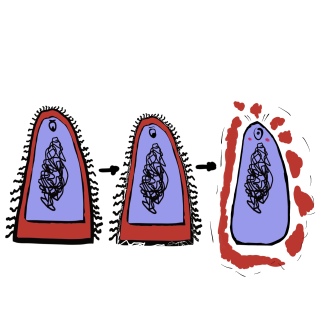
When you take penicillin, you’re sending waves and waves of these medical molecules to fight back the bacterial infection. After a few weeks, all that’s left are a few gutsy bacteria, they may be the most resilient, but even they cannot outlast the offensive power of penicillin much longer.
Unless…
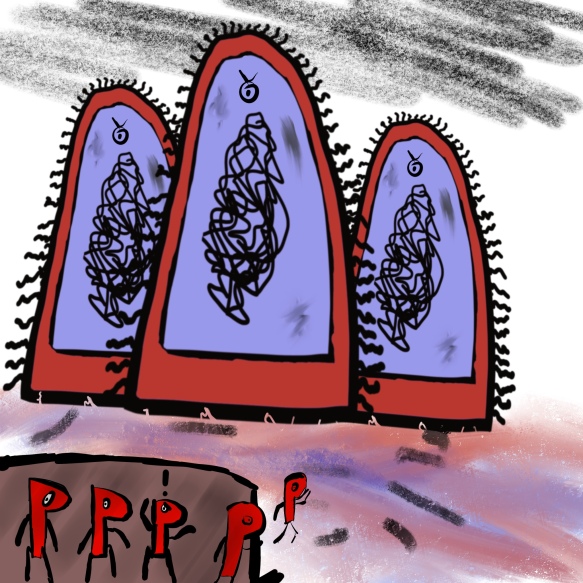
“Where are our reinforcements?” “Why hasn’t the general sent any?”
The general here, of course, is you. Every year thousands of people leave their antibiotic courses unfinished in the UK. Even though there are not enough bacteria for you to feel ill, they still exist inside you, and you’ve just given them the respite they need.
So why is this a problem? There are only a few bacteria left, and you don’t feel ill anymore! If it starts to get bad again you can just go back to the doctor for more medicine if you get ill, right? Unfortunately not. If the disease returned a month or so later, you may find the same treatment will not be as effective.
It turns out that the few remaining bacteria the first time had survived because they, out of all the other bacteria, were the only ones to possess a molecule called B-lactamase, which breaks down penicillin, keeping the transpeptidase builders safe.
I did not want to show graphic molecule on molecule violence, so use your imagination to what happens to penicillin after this.
Not only that, but this time all of the bacteria, not just the strongest few, have this ability. How can this be?
The issue comes from the way bacteria multiply. They divide. If the penicillin course remains unfinished, all bacteria in the infection will then be children of the remaining penicillin-breaking bacteria. Bacteria that have already proved their resistance and resilience. Some bacteria can divide into two every half an hour, growing from just a single cell to over a million in only twelve hours.
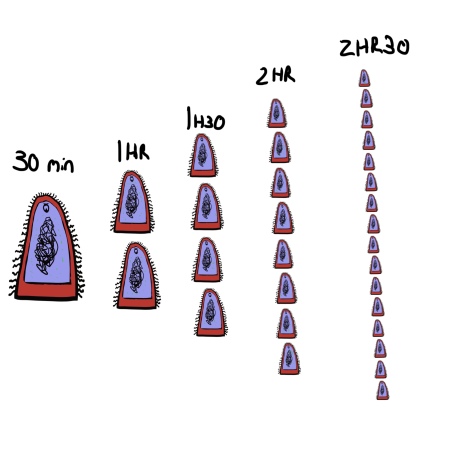
The bacteria don’t actually get smaller, its just the only way to fit them all in the picture.
This is obviously a problem on an individual level, but the resistance dilemma does not end there. Bacteria do not only transfer their ability when they divide, but can also transfer this ability to other nearby bacteria, even if they aren’t the same species. This is because they have a small circle of DNA encoding genes for this resistance. This little genetic instruction manual is passed between close-by bacteria, spreading the knowledge of how to prevent the damage caused by penicillin.
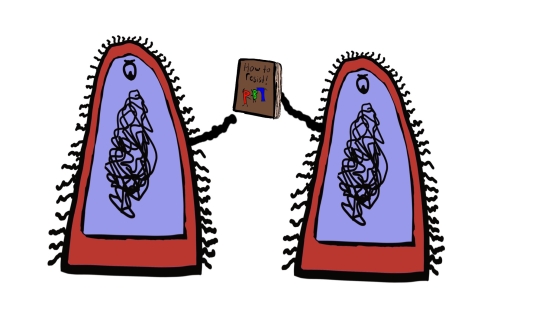
Never has a book club been so terrifying.
The combination of dividing bacteria and this trading of information means that anywhere with a high number of people, antibiotics and infectious diseases is going to be on the front line of this resistance war. Hospitals now have to fight against strains of bacteria that are resistant not just to penicillin, but to many different drugs. MRSA, the super-bug, is short for Methicillin-Resistant Staphylococcus Aureus, and has been a threat to patients both hospital bound and visiting for years. It is the stronger and harder version of the bacteria you see fighting the penicillin above. Over 44,000 people die of sepsis a year in the UK (more than lung cancer), and many of these are due to antibiotic-resistant infections.
This is why there are so many guidelines in place to help combat this growing resistance. Antibiotics are only given when absolutely necessary, and only in bacterial infections. If you have a cold or cough, these may be viral infections. Viruses hide inside our own cells, reproducing there, so antibiotics will have no effect at all. It would be like treating a splinter with a bandage, you aren’t going to get anything out and it’ll most likely just make things worse.
In terms of what you can do, it is advised that you take all of the medication the doctor gives you, and especially for the length of time the medical professional states. This is to ensure you finish off the strongest of the bacteria and avoid creating even more resistant strains. Feel free to discuss why this is with your doctor, they’re normally more than happy to talk about it.
There is another problem, not just outside of hospitals, but outside of humans altogether. Livestock. The World Health Organisation (WHO) states that in some countries over 80% of antibiotic use is in animals. Many farms across the world including Europe and the US are still using antibiotics in animals even when they are not ill. This sort of preventative care would be devastating for humans, and yet this is occurring in food that ends up in our bodies anyway. Marks and Spencer’s took an important step to help combat this less than a week ago, stating they will begin to publish data on antibiotics used in their supply chain. This will hopefully encourage others to do the same, and allow consumers to see what is being put into their food.

“I hear you work for big farmer”
These bacteria have no concerns jumping from livestock to humans and there were already cases last year of MRSA existing in livestock within Europe. We are using the same drugs on animals as we use ourselves, meaning we are sacrificing their potential medical use even quicker than necessary.
We’re putting all of our eggs in one basket, and we’re running out of eggs.
The second World health organisation (WHO) World Antibiotic Awareness week has just passed (14th-20th November). This annual event aims to help educate people, both public and professional, on the risks of antibiotic overuse. WHO are also advising farmers to only use antibiotics that are categorised as “least important to human health” in livestock.
In terms of what we as individuals can do, we can, on a personal level, ensure that we follow the doctor’s advice with how often we take our antibiotics, and advise everyone else that they do the same. We can spread the WHO’s message that current antibiotic use in animals is a ticking clock, and if it is not more carefully controlled we stand to lose a precious medical resource.
When they are turned to sparingly, and used appropriately, antibiotics can be an extremely effective tool against infections that would have previously been fatal. But without this control, we may risk living in what WHO describes as “the post-antibiotic era”. Like TV, phones and the internet, most of us have not experienced a world where antibiotics don’t exist, and one glance at history tells us that we don’t ever want to.
***
If you want to read more about how genes affect humans as well as cells, read my previous blog: “Genetics: The Real Book of Life.“
Interesting read Joe if you’ve not seen the following by Lee & Ventola,
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4378521/
It’s worth a read
All the best
Garry
LikeLike
Hi Joe, I really loved reading this and enjoyed the illustrations !
I would love to use this as patient information in our surgery and hope that is ok with you, looking forward to your next piece of work , regards Kerstin
LikeLike
Hi Kerstin,
Thank you you so much, you are welcome to distribute the material wherever you want as long as the web address is on there somewhere so people know where to find more. Hope it helps!
LikeLike